Important Traffic Notice: Due to road construction on Southwest Highway directly in front of our Chicago Ridge location, traffic may be heavier than usual. Please plan ahead and allow extra time to arrive safely. Thank you for your patience and understanding!
Before new cancer treatments can be made widely available to the general public, they must first be proven safe and effective. Clinical trials make this possible.
Clinical trials are research studies that involve people. Through clinical research studies, doctors prove new treatments to be effective and find new ways to improve upon current treatments as well as the quality of life for people with cancer. If you or a loved one need treatment for cancer, clinical trials could be an option to consider.
Affiliated Oncologists is an established research leader in communities across the greater Chicago area and offers clinical trials through Sarah Cannon Research Institute (SCRI), one of the world’s leading oncology research organizations conducting community-based clinical trials. SCRI has contributed to pivotal research that has led to the majority of new cancer therapies approved by the FDA today.

Our research program, SCRI at Affiliated Oncologists, provides access to the latest clinical trial options to people facing cancer and is part of an expansive research network with the goals of advancing therapies for patients and transforming cancer care within the community, where the majority of people seek cancer treatment. At any given time, Affiliated Oncologists offers clinical treatment options for various disease types, including:
- Anal Cancer
- Biliary Cancer
- Bladder Cancer
- Breast Cancer
- Cervical Cancer
- Esophageal Cancer
- Fallopian Tube Cancer
- Gallbladder Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Stomach Cancer
- Testicular Cancer
- Uterine Cancer
- Vaginal Cancer
- Vulvar Cancer
For more information, call 708-424-9710 or view all clinical trials available at Affiliated Oncologists.
Why Cancer Research Trials are Important
Clinical trials are a key research tool for advancing medical knowledge and patient care. Without cancer research and clinical trial participants, most of the cancer treatments used today as standard treatments would not be available. Cancer clinical trials are important because they provide patients with access to promising new treatments, allowing them to contribute to meaningful advances in cancer research. They also help develop future FDA-approved therapies that may be more effective or better tolerated than today’s treatment options.
Types of Clinical Trials
There are four different types of clinical trials, which include:
Treatment trials, which test new drugs, medical procedures, or combinations of treatments
Prevention trials, which look at cancer risk and ways to reduce that risk by either doing something (called action studies), like making lifestyle changes, or taking something (called agent studies), such as medicines, vitamins, or minerals
Screening trials, which test new ways to find a disease early, when it may be more easily treated
Quality of life trials, which explore ways to improve comfort and quality of life for cancer patients
Clinical Trial Phases
Clinical trials are categorized into four phases, and your oncologist will explain the phase and what to consider before deciding to participate. Most clinical trials available through Affiliated Oncologists are Phase II, III, or IV. They focus on how well a treatment works, how it compares to standard care, and how it is best used. Phase IV trials take place after FDA approval to gather additional information on a treatment’s risks, benefits, and optimal use. Placebos are never used instead of standard cancer treatment, and if included, they are given alongside active therapy so patients continue to receive care.
Learn More About Clinical Trial PhasesHow Cancer Clinical Trials Can Benefit You
By participating in a clinical trial, you can access new research treatments before they are widely available. Those considering participation must first learn the key facts, such as the purpose, risks, and benefits of a clinical trial, a process called informed consent.
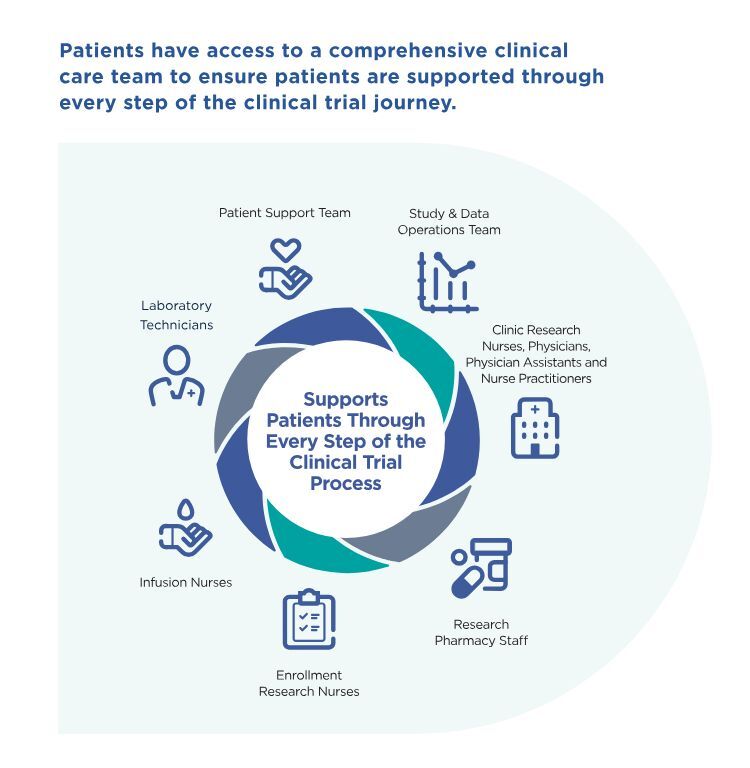
Additionally, a comprehensive clinical care team supports you at every step of the clinical trial journey. This includes:
- a patient support team
- a study and data operations team
- clinical research nurses, physicians, physician assistants, and nurse practitioners
- research pharmacy staff
- enrollment research nurses
- infusion nurses
- laboratory technicians
Keep in mind that cancer clinical trial participation is strictly voluntary. If you choose not to participate, you may withdraw your decision at any time and for any reason.
For most patients, clinical trials are not the first course of treatment that will be tried. But, as you progress through your cancer treatment process, you may qualify for a study that could benefit you and other cancer patients in the future.
Participation may also help others by contributing to the understanding and knowledge surrounding cancer and treatment.
For more information on clinical trials, our experts have answered the most frequently asked questions about clinical trial participation, or visit ClinicalTrials.gov or the National Cancer Institute. You may also contact us directly or learn more about the clinical trials available at Affiliated Oncologists.